Unveiling the Secrets of Isotopes: A Seating Map for Understanding the Elements
Related Articles: Unveiling the Secrets of Isotopes: A Seating Map for Understanding the Elements
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Unveiling the Secrets of Isotopes: A Seating Map for Understanding the Elements. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Unveiling the Secrets of Isotopes: A Seating Map for Understanding the Elements

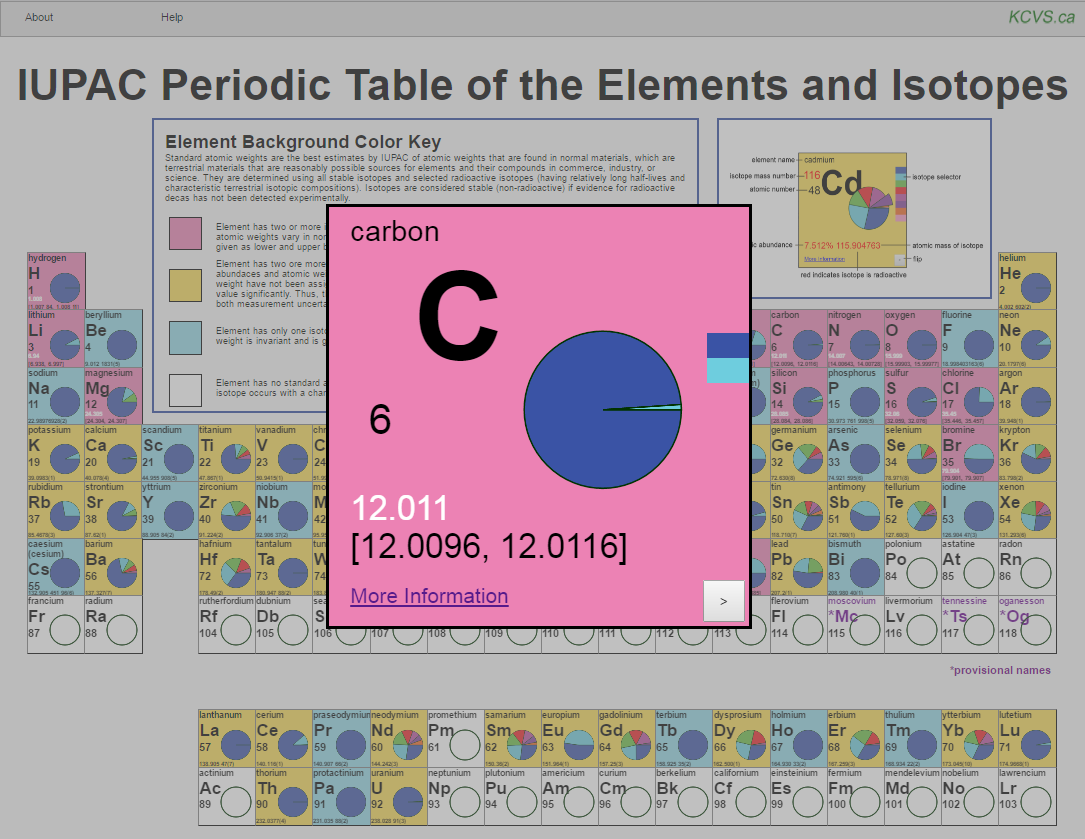
The world of chemistry is built upon the foundation of elements, the fundamental building blocks of all matter. Each element is defined by its atomic number, representing the number of protons within its nucleus. However, the story doesn’t end there. Atoms of the same element can exhibit subtle variations in their atomic mass, arising from differing numbers of neutrons in their nuclei. These variations give rise to isotopes, atoms of the same element with different numbers of neutrons.
Imagine a classroom filled with students. Each student represents an atom of a specific element. While all students share the same "atomic number" (i.e., the same number of protons), they might have different "atomic masses" due to varying numbers of neutrons. This analogy helps visualize the concept of isotopes, where the "seating map" of the classroom reflects the distribution of isotopes within a given element.
The Importance of the Isotope "Seating Map"
The "seating map" of isotopes, or the isotopic distribution, holds significant implications across various scientific disciplines. It serves as a powerful tool for:
- Understanding the evolution of the universe: Isotopic ratios provide insights into the processes that shaped the early universe, including the formation of stars and the synthesis of elements. For instance, the abundance of specific isotopes like carbon-14 and uranium-238 can be traced back to the Big Bang and subsequent stellar processes.
- Dating geological and archeological artifacts: Radioactive isotopes, like carbon-14 and uranium-238, undergo radioactive decay at a predictable rate. By measuring the ratio of parent isotope to daughter isotope, scientists can determine the age of rocks, fossils, and ancient artifacts. This technique, known as radiometric dating, has revolutionized our understanding of Earth’s history.
- Tracing environmental processes: Isotopes act as natural tracers, providing insights into various environmental processes. For example, the ratio of oxygen-18 to oxygen-16 in water molecules can reveal information about past climate conditions. Similarly, the isotopic composition of nitrogen in soil can indicate the presence of specific types of nitrogen-fixing bacteria.
- Medical diagnostics and treatments: Specific isotopes, like iodine-131 and technetium-99m, are used in medical imaging and treatment. These isotopes emit radiation that can be detected by scanners, allowing doctors to visualize internal organs and diagnose diseases. Certain isotopes are also employed in targeted therapy, delivering radiation directly to cancerous cells.
- Industrial applications: Isotopes play crucial roles in various industrial processes, including manufacturing, quality control, and research. For example, carbon-14 is used to study the wear and tear of machinery, while tritium is employed in the production of light sources and gauges.
Delving Deeper: Exploring the Isotope "Seating Map"
Understanding the "seating map" of isotopes requires exploring the following key concepts:
- Stable and Radioactive Isotopes: Not all isotopes are created equal. Some isotopes are stable, meaning their nuclei are not prone to decay. Others are radioactive, exhibiting a tendency to decay into different isotopes or elements. This decay process releases energy in the form of radiation.
- Isotopic Abundance: The relative abundance of each isotope within a sample of an element is known as its isotopic abundance. This abundance can vary depending on the source of the sample and the processes it has undergone.
- Mass Spectrometry: Mass spectrometry is a powerful technique used to determine the isotopic composition of a sample. It separates ions based on their mass-to-charge ratio, allowing scientists to identify and quantify the different isotopes present.
- Nuclear Reactions: Isotopes can be transformed into other isotopes or elements through nuclear reactions. These reactions involve the manipulation of the nucleus, often involving bombardment with particles or neutrons.
FAQs about Isotopes and their "Seating Map"
Q: What are the main differences between isotopes?
A: Isotopes of the same element differ in their number of neutrons, leading to variations in their atomic mass. This difference in neutron count can influence the stability and decay properties of the isotopes.
Q: How are isotopes used in everyday life?
A: Isotopes find applications in various fields, including medicine, agriculture, and industry. Examples include the use of carbon-14 in dating artifacts, iodine-131 in treating thyroid disorders, and uranium-235 in nuclear power generation.
Q: What are the potential hazards associated with isotopes?
A: Radioactive isotopes can pose health risks due to their ability to emit radiation. Proper handling and disposal are crucial to minimize exposure and ensure safety.
Q: How do scientists determine the isotopic composition of a sample?
A: Mass spectrometry is a widely used technique for determining isotopic composition. It separates ions based on their mass-to-charge ratio, allowing scientists to identify and quantify the different isotopes present.
Tips for Understanding the "Seating Map" of Isotopes
- Visualize isotopes using analogies: The classroom analogy can be helpful in understanding the concept of isotopes. Imagine students with the same number of protons but different numbers of neutrons, representing different isotopes of the same element.
- Focus on the key properties: Emphasize the differences in atomic mass, stability, and decay properties between isotopes.
- Explore real-world applications: Highlight the diverse applications of isotopes in various fields, from medicine to industry, to illustrate their practical relevance.
- Utilize resources: Explore online resources, textbooks, and scientific journals to deepen your understanding of isotopes and their "seating map".
Conclusion
The "seating map" of isotopes, or the isotopic distribution, provides a powerful framework for understanding the intricacies of the elements. By exploring the variations in neutron count and their impact on atomic mass, stability, and decay properties, scientists can unravel the secrets of the universe, date ancient artifacts, and harness the power of isotopes for various applications. This "seating map" serves as a testament to the complexity and elegance of the natural world, reminding us that even seemingly identical elements can harbor hidden variations that hold profound implications for our understanding of the universe and our place within it.




/Isotope-58dd6b415f9b5846830254ae.jpg)
Closure
Thus, we hope this article has provided valuable insights into Unveiling the Secrets of Isotopes: A Seating Map for Understanding the Elements. We appreciate your attention to our article. See you in our next article!